24 Hour Frozen Recovered Plasma
Seraplex can supply 24 Hour Frozen Recovered Plasma for both in vitro diagnostics and for plasma fractionation.
Seraplex sources 24 Hour Frozen Recovered Plasma from both community blood centers and hospital blood banks. This is plasma that cannot be used for transfusion, such as female donor plasma or male plasma that is not frozen within the 8 Hour time frame for making Fresh Frozen Plasma. The 24 Hour Frozen Recovered Plasma we collect from hospital blood banks is used for in vitro diagnostic manufacturing, while most of the 24 Hour Frozen Recovered Plasma collected from community blood centers is sent for plasma fractionation.
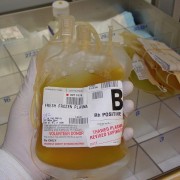
24 Hour Frozen Recovered Plasma is sourced from FDA registered centers is the United States and is clean and clear, with golden to straw color.
Recovered Frozen Plasma (Frozen within 24 hours)
Product # PS420
- Recovered plasma frozen from qualified human volunteer blood donors, collected at FDA registered Blood Establishments.
- Plasma is prepared from separated whole blood, or from plasma collected by apheresis, collected in FDA approved anticoagulant, processed under normal aseptic conditions, separated and frozen within 24 hours of collection, and stored at -20 C or colder.
- Anticoagulants
- CPD, CP2D, CPDA, and ACD anticoagulant may be used.
- Labeling
- Each unit labeled with the product description, unit number, anticoagulant, collection date and/or expiration date, and the name of blood establishment.
- Appearance
- Clear and golden colored, non-lipemic, free of hemolysis, and particulate matter.
No unusual discoloration or odor.
- Clear and golden colored, non-lipemic, free of hemolysis, and particulate matter.
- Included with each shipment:
- Signed Certificate of Compliance with the following information:
- Certification of testing.
- Each donor unit tested by an FDA licensed laboratory with FDA licensed test kits.
Test results Negative or Non-Reactive for HBsAg, anti-HBc, anti-HIV 1/2, anti-HTLV I/II,
anti-HCV, and Syphilis, HIV -1 RNA and HCV RNA. Results for other viral tests if performed such as HBV NAT, West Nile Virus and Chagas (T. cruzi) Negative or Non-Reactive. - Certification of Storage Temperature. The plasma has been maintained at -20 C or colder.
- Collection Data: A record listing the unit number, collection date and / or expiration date will accompany each shipment. Each unit will be cross-referenced to the box in which it is packed.
- Signed Certificate of Compliance with the following information:
- Packing
- Packed in boxes with insulation material and dry ice, if required.
- Shipping
- Depends on customer specification.
Transported to storage facility or final destination by freezer truck.
Can be air-freighted in containers with dry ice.
Next Day Delivery by Federal Express
- Depends on customer specification.
- Order Lead Time
- Depends on required volume. Contracts and Standing Orders available.
Please email us at info@seraplex.com or call (626) 792-9945 to inquire about pricing or to place an order.
Contact – Office Number
(626) 792-9945
